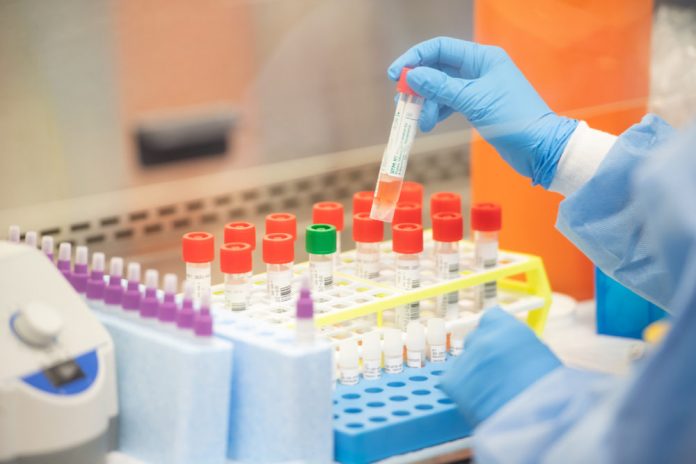
The Food and Drug Administration said Tuesday that it has issued its first emergency approval of an at-home collection kit for the coronavirus.
The kit allows people to collect their own sample and then send it to the company to be tested. The kit is produced by North Carolina-based LabCorp.
The collection kit will be first made available to healthcare workers and first responders who have symptoms of COVID-19, LabCorp said in a press release, but they added that they hope to make the tests available to consumers in “the coming weeks.”
The FDA approved the use of the nasal swab tests after granting a LabCorp request under emergency measures.