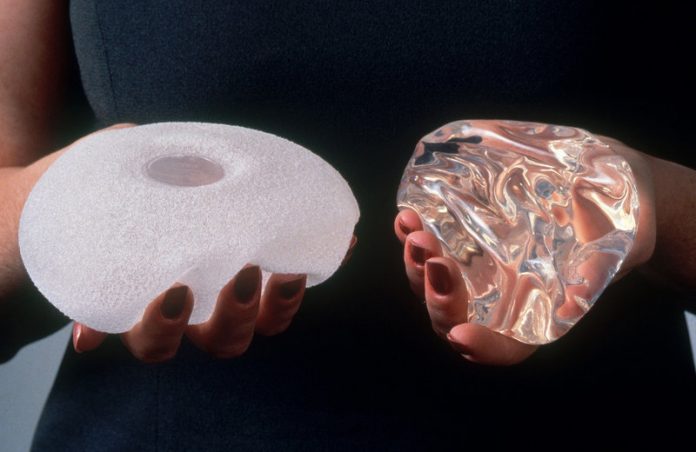
The United States Food and Drug Administration (FDA) issued a statement on Wednesday about the seriousness of the known link between breast implants and a rare cancer, anaplastic large cell lymphoma (ALCL). At least 457 women in the U.S. have been diagnosed with breast implant-associated lymphoma, also referred to as BIA-ALCL, and nine of those women have died. The FDA is basing these numbers on medical device reports related to ALCL that it has received since 2010.
Officials at the FDA wrote, “We hope that this information prompts providers and patients to have important, informed conversations about breast implants and the risk of BIA-ALCL.” Despite being associated with implants, ALCL is not breast cancer. It is a rare blood cancer, a non-Hodgkin’s lymphoma, and can spread throughout the body. As a supplement to its Wednesday statement, the FDA also published a letter asking health care providers to continue to report possible cases of breast cancer-related ALCL to the agency.
The FDA first sounded the alarm on BIA-ALCL back in 2011. The World Health Organization has noted that since the link was identified two decades ago, ALCL has been linked with both implants saline- and silicone-filled implants.
Additionally, implants are linked to a heightened risk of developing ALCL regardless of whether they are smooth or textured. In November, a renewed investigation into the link between textured breast implants and the rare lymphoma forced several companies that manufacture textured implants, including Allergen and Mentor, to state that despite risks, they would not cease production. The companies urged physicians to continue to inform patients of potential risks associated with the implants.