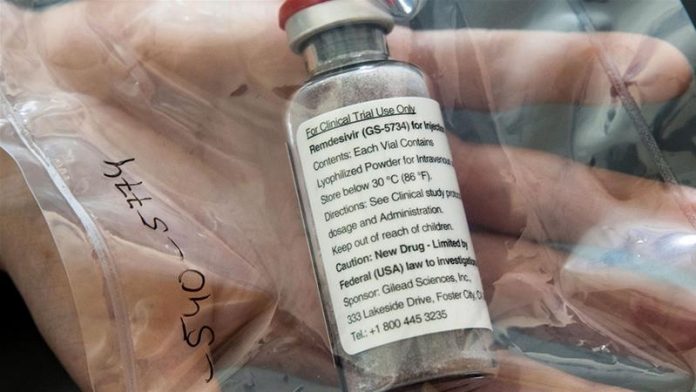
The maker of remesivir, an experimental drug that is reportedly showing promise for treatment of the coronavirus, is “moving very quickly with the FDA” to get approval, its CEO said Friday.
Daniel O’Day, of Gilead Sciences, told the “Today” show that the pharmaceutical company has already increased its treatment courses from 5,000 to 100,000 and hopes to make 1 million available by the end of the year.
The drug has shown some promise for severely ill patients. Early results from one study found patients who received remdesivir had a 31% faster recovery time than those who received a placebo.
“This is a medicine that’s really for the most severe patients. This is for hospitalized patients, this is for patients that have really progressed …” he said. “What we see here is really, of course not a cure, but a very, very significant and important treatment for patients.”